How Do You Know Monocot and Dicot Are Basic or Acidic? After Pcr
- Research article
- Open up Admission
- Published:
Monocot and dicot MLO powdery mildew susceptibility factors are functionally conserved in spite of the evolution of grade-specific molecular features
BMC Plant Biological science book 15, Article number:257 (2015) Cite this commodity
Abstract
Background
Specific members of the plant Mildew Locus O (MLO) protein family act every bit susceptibility factors towards powdery mildew (PM), a worldwide-spread fungal illness threatening many cultivated species. Previous studies indicated that monocot and dicot MLO susceptibility proteins are phylogenetically divergent.
Methods
A bioinformatic approach was followed to study the type of evolution of Angiosperm MLO susceptibility proteins. Transgenic complementation tests were performed for functional analysis.
Results
Our results bear witness that monocot and dicot MLO susceptibility proteins evolved grade-specific conservation patterns. Many of them appear to be the effect of negative selection and thus are probable to provide an adaptive value. Nosotros likewise tested whether different molecular features between monocot and dicot MLO proteins are specifically required by PM fungal species to cause pathogenesis. To this aim, we transformed a tomato mutant dumb for the endogenous SlMLO1 gene, and therefore resistant to the tomato plant PM species Oidium neolycopersici, with heterologous MLO susceptibility genes from the monocot barley and the dicot pea. In both cases, we observed restoration of PM symptoms. Finally, through histological observations, we demonstrate that both monocot and dicot susceptibility alleles of the MLO genes predispose to penetration of a non-adjusted PM fungal species in found epidermal cells.
Conclusions
With this study, we provide insights on the evolution and office of MLO genes involved in the interaction with PM fungi. With respect to breeding research, we show that transgenic complementation assays involving phylogenetically distant plant species tin be used for the characterization of novel MLO susceptibility genes. Moreover, nosotros provide an overview of MLO poly peptide molecular features predicted to play a major role in PM susceptibility. These represent ideal targets for future approaches of contrary genetics, addressed to the selection of loss-of-function resistant mutants in cultivated species.
Background
The found Mildew Locus O (MLO) gene family codes for proteins harboring seven transmembrane domains and a calmodulin-binding site, topologically reminiscent of metazoan and fungal K-poly peptide coupled receptors (GPCRs) [1]. Following the completion of plant genome sequencing projects, a number of homologs varying from 12 to 19 has been identified in the MLO cistron families of diploid species, namely Arabidopsis, rice, grapevine, cucumber, peach, woodland strawberry and sorghum [1–6].
Specific homologs of the MLO gene family act equally susceptibility factors towards fungi causing the powdery mildew (PM) illness, worldwide spread and causing astringent losses in agricultural settings. Inactivation of these genes, through loss-of function mutations or silencing, indeed results in resistance (referred to equally mlo-based resistance) in several plant species [7]. The showtime MLO gene described as required for PM pathogenesis was barley HvMLO [8, ix]. Since and so, MLO susceptibility genes have been functionally characterized in rice (OsMLO3), wheat (TaMLO_A1 and TaMLO_B1), Arabidopsis (AtMLO2, AtMLO6 and AtMLO12), lycopersicon esculentum (SlMLO1), pepper (CaMLO2), tobacco (NtMLO1), pea (PsMLO1), lotus (LjMLO1) and barrel clover (MtMLO1) [10–17].
Defense mechanisms involved in mlo-based resistance preclude fungal penetration in epidermal cells and are associated with the formation of cell wall appositions, referred to every bit papillae [11]. Similar pre-penetration defense measures also accept place in not-host resistance, following the interaction between PM fungal species and institute species beyond their host range. Consistent with the hypothesis of involvement of MLO genes in non-host resistance, loss of role of HvMLO in the interaction between barley and the wheat PM fungus Blumeria graminis f. sp. tritici is associated with decreased rate of penetration and lower incidence of epidermal cell death, the latter being a mail service-penetration defense mechanism [18, 19].
Several studies have been addressed to the label of regions of relevance for the functionality of MLO proteins. Multiple alignments have pointed out the occurrence of residues highly conserved inside the whole MLO family, which were therefore predicted to provide a mutual protein structural scaffold [12, 20]. In addition, the occurrence of residues and motifs specifically conserved in putative orthologs of barley HvMLO has been reported [9]. Finally, functionally important residues for MLO susceptibility proteins have been inferred by the association of naturally occurring and induced mutations with partial or consummate PM resistance [11, 12, 21–25].
In our previous studies, we showed that phylogenetically related dicot MLO genes of the same botanic family unit are conserved for their function as a susceptibility gene to PM [six, xvi]. Notably, monocot and dicot MLO proteins involved in PM susceptibility group in conspicuously separated phylogenetic clades (east.g. [ii, ix]). Here, we show that the evolution of Angiosperm PM susceptibility genes led to the fixation of class-specific molecular traits. Many of them appear to be the result of negative selection. By means of transgenic complementation assays, nosotros demonstrate that, despite having different conservation patterns, monocot and dicot MLO susceptibility genes are essentially conserved with respect to functional features having a part in interactions with PM fungi. Consequences of our findings for plant convenance research are discussed.
Results
Form-specific molecular features of Angiosperm MLO homologs required for PM susceptibility
Previous studies indicated that dicot and monocot MLO proteins with a putative or ascertained role in susceptibility to PM fungi group in ii different phylogenetic clades (e.g. [2, 9]). This was confirmed by performing a new UPGMA-based phylogenetic assay involving all the 12 MLO homologs which take been until recently functionally related to PM susceptibility (Fig. 1). Aiming to detect molecular features responsible for such phylogenetic departure, the aforementioned MLO homologs were used equally dataset for protein multiple alignment (Fig. 2). Notably, this led to the identification of 41 alignment positions in which residues invariable throughout dicots are absent in monocots, and 84 alignment positions in which residues changeless throughout monocots are absent-minded in dicots. In 44 alignment positions, class-specific residues are replaced in the other class with residues having different properties, according to the chemical features of their side-chain group (hydrophobic, polar basic, polar acidic and polar uncharged).
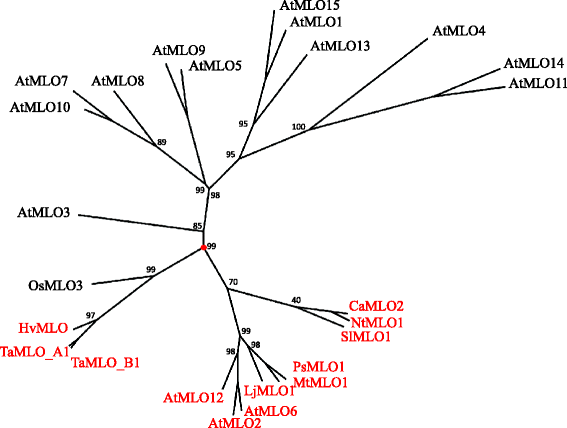
Unrooted radial phylogenetic tree of MLO powdery mildew susceptibility proteins. The tree includes, in ruby, all the monocot and dicot MLO homologs shown to be required for powdery mildew susceptibility (Arabidopsis AtMLO2, AtMLO6 and AtMLO12, love apple SlMLO1, pepper CaMLO2, tobacco NtMLO1, pea PsMLO1, lotus LjMLO1, barrel clover MtMLO1, barley HvMLO, wheat TaMLO_B1 and TaMLO_A1b and rice OsMLO3), and the remaining homologs of the Arabidopsis AtMLO family. Numbers at each node correspond bootstrap support values (out of 100 replicates)
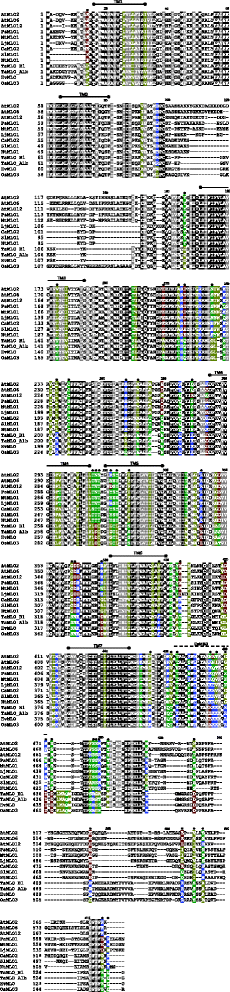
Multiple alignment of MLO powdery mildew susceptibility proteins. The dataset is composed of all the monocot (barley HvMLO, rice OsMLO3, wheat TaMLO_B1 and TaMLO_A1b), and dicot (Arabidopsis AtMLO2, AtMLO6 and AtMLO12, tomato plant SlMLO1, pepper CaMLO2, tobacco NtMLO1, pea PsMLO1, lotus LjMLO1 and barrel clover MtMLO1) MLO homologs shown to act as powdery mildew susceptibility factors. The positions of the vii MLO transmembrane domains (TM1-TM7) and the calmodulin binding domain (CaMBD) are identical to the ones reported by Feechan et al. [2], Functional Constitute Biology, 35: 1255–1266. Black color indicates alignment positions in which invariable residues are present. Grey color indicates alignment positions which do non comprise course-specific residues and are conserved with respect to biochemical backdrop. Other colors point alignment positions in which in that location are course-specific residues in monocots, dicots, or both: yellow indicates hydrophobic residues (Thousand, A, V, 50, I, F, W, M, P); bluish indicates polar basic residues (K,R,H); cherry indicates polar acidic residues (D, E); dark-green indicates polar uncharged residues (S, T, C, Y, Due north, Q). Black dots highlight 44 alignment positions in which grade-specific residues are substituted in the other grade by residue(southward) having different biochemical properties
Adaptive relevance of class-specific molecular features supported by evolutionary assay
In order to make inference on the evolutionary events leading to the to a higher place mentioned form-specific molecular features, we performed a codon-based Single-Likelihood Ancestor Counting (SLAC) assay on the difference of nonsynonymous to synonymous substitutions per nonsynonymous and synonymous sites (dN-dS). Tests were conducted to predict the evolution of each codon: neutral/dN = dS or negative (purifying)/dN < dS. Nosotros decided to restrict the analysis to a panel of nine dicot MLO susceptibility genes, as only four monocot MLO homologs have been so far associated with PM pathogenesis and the dN-dS analysis can provide significant results only when using a sequence dataset which is not too minor. We found 130 codons under significant negative selection, coding for amino acids scattered throughout MLO protein domains. Among the 130 codons, 27 are translated into form-specific residues, which are therefore predicted to provide an adaptive value (Additional file one).
Functional conservation of monocot and dicot MLO susceptibility genes
We tested whether different molecular features between monocot and dicot MLO proteins are specifically required past PM fungal species infecting either one or the other grade of Angiosperms. To this aim, nosotros developed two constructs for the transgenic expression of a monocot (barley HvMLO) and a dicot (pea PsMLO1) MLO gene in the tomato Slmlo1 line, which is homozygous for a loss-of-function mutation in the endogenous factor SlMLO1 and therefore resistant to the tomato PM fungus Oidium neolycopersici. We reasoned that complementation and restoration of PM symptoms would take occurred only in case of functional conservation between SlMLO1 and any of the two tested transgenes. In full, nineteen 35S::PsMLO1 and 20 35S::HvMLO transformants were obtained. In both cases, eighteen individuals were obtained showing variable transgene expression levels. For each construct, three Tane plants displaying high transgene expression (35S::PsMLO1-iv,−six and−vii and 35S::HvMLO-9,−10 and−xv) were self-pollinated to generate T2 families (Additional file 2 ). Ten individuals from each T2 family were tested for the presence or the absenteeism of the transgene and challenged with O. neolycopersici. Transgenic individuals of the iii T2 families overexpressing PsMLO1 (35S::PsMLO1_(+)) displayed PM symptoms with an average D.I. (disease index) score ranging from two.87 to 2.92. Transgenic individuals of the three T2 families overexpressing HvMLO (35S::HvMLO_(+)) showed an boilerplate D.I. score ranging from 1.viii to two.4. In dissimilarity, all not-transgenic 35S::PsMLO1_(−) and 35S::HvMLO_(−) T2 individuals displayed, similar to the Slmlo1 plants, hardly any fungal growth (Fig. 3 and Additional file iii). For transgenic plants of the three 35S::HvMLO T2 families, positive correlation was found between boilerplate D.I. and transgene expression level of respective Tane plants (Fig. 3 and Additional file 2 and 3). Together, these results betoken that monocot and dicot MLO susceptibility genes are functionally conserved with respect to molecular features required for PM pathogenesis.
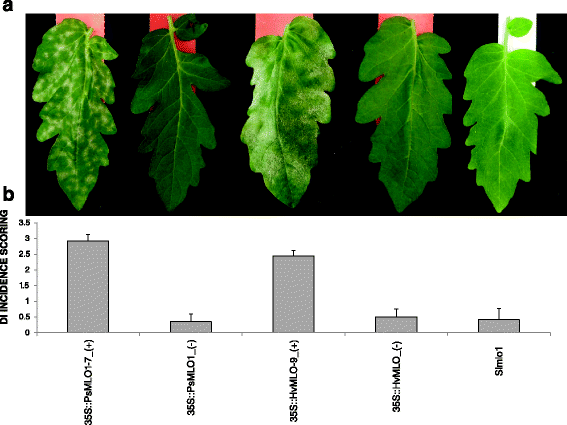
Transgenic overexpression of pea PsMLO1 and barley HvMLO in the tomato mutant line Slmlo1. Panel a shows the phenotypes of two selected individuals of the T2 family 35S::PsMLO1-7, segregating for the presence (starting time from the left) or the absence (second from the left) of the transgene, two selected individuals of the T2 family 35S::HvMLO-ix, segregating for the presence (third from the left) or the absence (second from the right) of the transgene, and one individual of the Slmlo1 line (beginning from the right), in response to the tomato powdery mildew fungus Oidium neolycopersici. Panel b from left to right shows average illness index (DI) values relative to transgenic plants (+) of the 35S::PsMLO1-seven T2 family, non-transgenic plants (−) of three Tii families segregating for the 35S::PsMLO1 construct, transgenic plants of the 35S::HvMLO-nine Tii family unit, non-transgenic plants of three T2 families segregating for the 35S::HvMLO construct and the Slmlo1 line. Standard deviation confined refer to vi 35S::PsMLO1_(+) individuals, nine 35S::HvMLO_(+) individuals, seven PsMLO1_(−) individuals, 7 HvMLO_(−) individuals and x Slmlo1 individuals
Functional conservation of monocot and dicot MLO susceptibility genes in non-host interactions
We next investigated whether functional conservation betwixt monocot and dicot MLO homologs also holds true in not-host plant-PM interactions. To this aim, we used the PM species B. graminis f.sp. hordei (Bgh) to inoculate plants of the Slmlo1 mutant line, the cultivar Moneymaker (MM), carrying wild-type SlMLO1, and ii of the 35S::HvMLO T2 families (35S::HvMLO-9 and −10, previously described in Fig. three, Additional file 2 and iii). Bgh is an adapted PM on barley and a non-adjusted PM to tomato. In the Slmlo1 line, 75.iv % of infection units were associated with papilla formation and 24.vi % with cell decease response (Fig. iv). Compared with the Slmlo1 line, transgenic 35S::HvMLO-9 Ttwo plants displayed a lower level of papilla formation (31.3 %) and a higher level of cell expiry response (68.7 %). In MM, papilla formation and cell death occurred at a rate similar to the one in 35S::HvMLO-9 plants (14.6 % and 85.four %, respectively). Taken together, this body of show indicates that both HvMLO and SlMLO1 predispose to the penetration of a non-host pathogen.
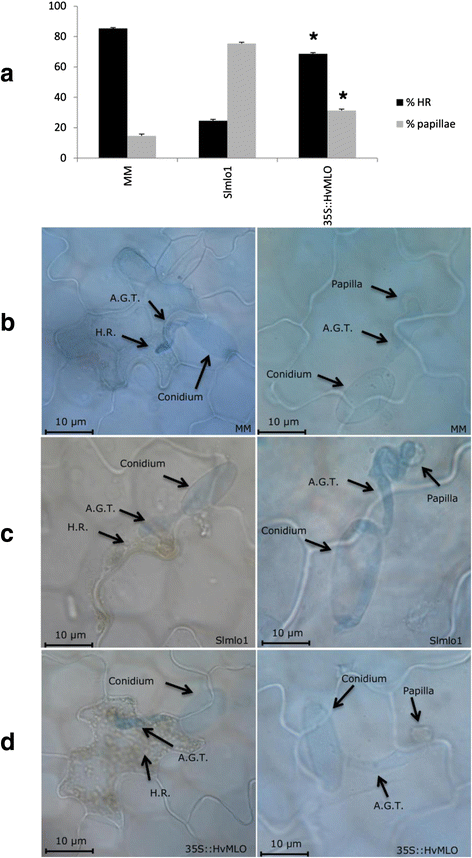
Functional conservation of SlMLO1 and HvMLO in the tomato plant/Blumeria graminis f.sp. hordei (Bgh) interaction. Console a shows the ratio of penetrated and non-penetrated epidermal cells, assessed in function of infection units showing hypersensitive response (H.R.) and papillae, respectively, in the post-obit genotypes: the mlo mutant line Slmlo1; the cultivar MM, with a like genetic background and carrying wild-type SlMLO1; transgenic plants of a T2 family overexpressing barley HvMLO in the Slmlo1 genetic background (35S::HvMLO-9). Panel b, c and d show, in the same genotypes, fungal structures (conidiospore and appressorium germination tube -A.G.T.-) and cellular events (the formation of papillae and H.R.) arresting fungal growth before and afterward penetration, respectively
Discussion
The functional characterization of MLO homologs involved in PM susceptibility is of great involvement for bones research on plant-microbe interactions as well as for plant breeding, as loss-of-function genotypes could exist conveniently used to introduce durable and broad-spectrum resistance in cultivated species [7]. Results of previous investigations indicated that mlo-based resistance in a certain plant species can be lost by the heterologous expression of MLO susceptibility genes from related species of the same botanical family. Indeed, restored susceptibility has been observed in barley HvMLO mutants transformed with wheat TaMLO_B1 and rice OsMLO3, likewise every bit in pea PsMLO1 mutants expressing lotus LjMLO1 or butt clover MtMLO1[12, thirteen]. Recently, similar prove was shown on lycopersicon esculentum SlMLO1 mutants transformed with pepper CaMLO2 or tobacco NtMLO1 [16, 17]. Hither, we investigated whether complementation tin besides occur by transferring MLO genes from more evolutionary divergent constitute species. We found that, in a tomato mlo mutant groundwork, transgenic expression of a MLO susceptibility factor from pea (a distantly related dicot species) and barley (a monocot species) is sufficient to re-establish PM susceptibility (Fig. 3 and Boosted file iii). This finding indicates that, despite phylogenetic distance and the development of peculiar molecular traits (Fig. 1 and 2), monocot and dicot MLO proteins are essentially conserved with respect to features involved in the interaction with PM pathogens. In support of this decision, nosotros show that the monocot gene HvMLO and the dicot cistron SlMLO1 both enhance penetration of the non-adjusted pathogen B. graminis f.sp. hordei compared to a tomato mlo-mutant (Fig. four). Moreover, after reviewing scientific literature, we found that only i out of thirty MLO protein substitutions and so far associated with PM resistance involves a class-specific residue (a monocot-specific alanine residue in position 350 of the alignment in Fig. two) (Table one) [22]. The aforementioned residue is replaced in dicots by a glycine (sharing like non-polar chemic properties of alanine, Table 1), indicating that, in this case, form-specific conservations are non associated with important changes in poly peptide structure or office.
We cannot exclude that course-specific traits might have pocket-sized effects on interactions with PM fungi. Indeed, past comparing three independent T2 families for each construct, we found that that overexpression of PsMLO1 results in higher D.I. alphabetize scores than the ane of HvMLO (Fig. 3 and Additional file iii). Clearly, complementation tests with several other monocot and dicot transgenes could help to answer this question.
Through the analysis of the dN-dS difference, we provide evidence for negative option interim on several class-specific residues, which are thus likely to play a major adaptive role (Additional file 1). However, as mentioned earlier, transgenic complementation tests betoken that these class-specific residues are not crucial for the outcome of the interaction betwixt plants and PM pathogens. Possibly, some of the class-specific residues identified in this written report might underlie roles which are non related with the interaction with PM fungi. The implication of MLO susceptibility proteins in other physiological processes would explain why, in spite of being required for pathogenesis, they have been not excluded by evolution. With this respect, it is worth to mention that PM resistance in Arabidopsis and barley mlo mutants has been associated with the consecration of leafage senescence, a pleiotropic phenotype [xi].
We show that MLO homologs required for PM pathogenesis tin can complement a mlo mutant background in transgenic assays, irrespective of the phylogenetic distance between the donor and the recipient species (Fig. 3). This would be of great advantage in guild to test the function of candidate MLO susceptibility genes which are currently beingness identified by several authors across cultivated species [4, 5]. Moreover, nosotros provide an overview of MLO protein regions which are under negative selection and thus are expected to exist of functional relevance. These regions represent platonic targets to select loss-of-function mutants resistant to the PM disease. With this respect, breeders may apply diverse tools, such equally conventional targeted mutagenesis approaches of TILLING (targeted induced local lesions in genomes) or avant-garde technologies of genome editing, based on zinc finger nucleases (ZFNs), amassed regularly interspaced brusque palindromic repeat (CRISPR) and transcription activator-like effector nucleases (TALEN) [26–28].
Determination
This work provides insights on the evolution and role of Angiosperm MLO susceptibility genes. We bear witness that complementation assays like to those carried out in this written report are suitable for future activities aimed at the label of novel PM susceptibility factors across cultivated species. Moreover, we indicate a series of factor targets for the pick of loss-of-role mlo resistant mutants.
Methods
Bioinformatic analyses
The post-obit MLO proteins, experimentally shown to exist required for PM susceptibility, were used every bit dataset for CLUSTAL alignment using the CLC sequence viewer software (http://world wide web.clcbio.com/products/clc-sequence-viewer/): Arabidopsis AtMLO2 [GenBank: NP172598], AtMLO6 [GeneBank: NP176350] and AtMLO12 [GeneBank: NP565902], tomato SlMLO1 [GeneBank: NP001234814], pea PsMLO1 [GeneBank: ACO07297], pepper CaMLO2 [GeneBank: AFH68055], lotus LjMLO1 [GeneBank: AAX77015], barrel clover MtMLO1 [GeneBank: ADV40949], barley HvMLO [GeneBank: P93766], rice OsMLO3 [GeneBank: AAK94907], wheat TaMLO_B1 [GeneBank: AAK94904] and TaMLO_A1b [GeneBank: AAK94905]. The alignment was given to Geneious v8 software (http://world wide web.geneious.com, [29] ), to highlight amino acids with different polarity, and the online web service Phylogeny.fr (http://www.phylogeny.fr/) to construct an unrooted radial phylogenetic tree.
In social club to make predictions on the type of evolution (negative or neutral) of class-specific molecular features, all the above mentioned dicot MLO susceptibility genes were used as dataset for a codon-based evolutionary assay based on the difference of nonsynonymous-to-synonymous substitutions per nonsynonymous and synonymous sites (dN/dS). This was performed by using the Unmarried-likelihood Ancestor Counting (SLAC) method implemented by the Datamonkey web server (www.datamonkey.org). The default p-value of 0.1 was taken equally threshold to call codons under significant negative pick.
Isolation and cloning of full-length PsMLO1 and HvMLO
Full RNAs from pea (cultivar Sprinter) and barley (cultivar Maythorpe) were isolated past using the RNeasy plant mini kit (Qiagen), and respective cDNAs were synthesized by using the SuperScript Three starting time-strand synthesis kit (Invitrogen) and the oligo(dT)20 primer. Specific primer pairs, named PsMLO1-Fw/PsMLO1-Rev and HvMLO-Fw/HvMLO-Rev (Additional file 4: Table S2) were manually designed in order to dilate the PsMLO1 and HvMLO full-length coding sequences, respectively. PCR reactions were performed past using the high-fidelity Phusion Dna polymerase (New England Biolabs) and an annealing temperature of 55 °C. Amplicons were ligated into the Gateway-compatible vector pENTR D-TOPO (Invitrogen) and cloned into the E. coli One Shot® TOP10 cells (Invitrogen), according to the manufacturer's instructions. After selecting positive colonies past colony PCR, using the two cistron-specific primer pairs to a higher place mentioned, recombinant plasmids were extracted and their inserts were sequenced. A unmarried colony for each construct was selected, in which the inserts resulted to take sequences identical to those of HvMLO and PsMLO1 deposited in the NCBI database.
Generation and functional characterization of transgenic SlMLO1 mutant tomato plants expressing PsMLO1 and HvMLO
Post-obit the manufacturer instructions (Invitrogen), cloned HvMLO and PsMLO1 gene sequences were inserted past LR recombination into the binary plasmid vector pK7WG2, which harbors the 35S Cauliflower Mosaic Virus (CaMV) promoter and the marker gene nptII for kanamycin resistance choice. Plasmids were then transferred to E. coli and positive colonies were screened by colony PCR and sequencing, as previously mentioned. Finally, recombinant vectors were extracted and transferred to the AGL1-virThousand strain of A. tumefaciens by electroporation.
The transformation of the tomato ol-2 mutant line, carrying a loss-of-part mutation of the PM susceptibility gene SlMLO1, was performed according to the methods described by [6] and [16]. The evaluation of the expression levels of PsMLO1 and HvMLO in T1 plants was carried out by existent-time qPCR using the primer pairs qPsMLO1-Fw/qPsMLO1-Rev and qHvMLO-Fw/qHvMLO-Rev (Additional file 4). A primer pair designed on the elongation factor 1α factor (qEF-Fw/qEF-Rev) was used for relative quantification (Boosted file 4).
Functional label of host and non-host interactions
For each of the two transgenes above mentioned, three T1 individuals showing the highest expression levels were allowed to self-pollinate, resulting in a total of half-dozen Ttwo families. Individuals of each family were assayed for the presence/absenteeism of the overexpression construct by means of PCR, using the primer pairs NPTII_Fw/ NPTII_Rev and 35S-Fw/35S-Rev designed on the nptII marker gene and the 35S promoter, respectively (Additional file four). Ten resistant Slmlo1 plants carrying the loss-of-function SlMLO1 allele and 10 individuals of each family unit were challenged with an isolate of the love apple PM mucus O. neolycopersici maintained at the Plant Breeding Department of the University of Wageningen, The netherlands. Inoculation was performed as described by [30], spraying 4 weeks-one-time plants with a suspension of conidiospores obtained from freshly sporulating leaves of heavily infected plants and adapted to a final concentration of four × 104 spores/ml. Inoculated plants were grown in a greenhouse compartment at 20 ± two °C with lxx ± fifteen % relative humidity. Illness evaluation was visually carried out 15 days after inoculation, based on the presence of illness signs on the third and fourth leaf, co-ordinate to the scale from 0 to 3 reported by [ten].
For the functional label of a non-host interaction, seeds from 1 of the three 35S::HvMLO Tii families previously tested were surface-sterilized and sown on half-strength Murashige and Skoog (MS) agar supplemented with 50 mg/ml kanamycin for selection of transgenic plants. Seeds were left for ii days at 4 °C and so transferred to a growing bedchamber for 10 days. Five transgenic seedlings were transplanted in pots and transferred to a greenhouse compartment. Three barley plants of the PM susceptible cultivar Manchuria, five Slmlo1 plants and 5 MoneyMaker plants were used as controls. An isolate of B. graminis f. sp. hordei (Bgh) nerveless in Wageningen (Wag.04) was used for the inoculation. This was performed by rubbing Manchuria leaves heavily infected with Bgh on the third tomato leafage. After 72 h, in which inoculated plants were kept in a climate chamber at 20 °C, 16 h of light/day and 70 % RH, a four cmii segment was cutting from the inoculated leaves (third leaf). Three samples were taken from 3 plants of each genotype.
Each leafage segment was bleached is a 1:iii (v/v) acerb-acid/ethanol solution and 48 h later stained in 0.005 % Trypan Blueish every bit described by [31]. The rate of fungal penetration was estimated by the frequency of infection units showing epidermal cell death. For each genotype, 3 biological replicates were considered, considering at to the lowest degree 100 infection units.
References
-
Devoto A, Hartmann HA, Piffanelli P, Elliott C, Simmons C, Taramino G, et al. Molecular phylogeny and evolution of the plant-specific 7-transmembrane MLO family. J Mol Evol. 2003;56(ane):77–88.
-
Feechan A, Jermakow AM, Torregrosa L, Panstruga R, Dry IB. Identification of grapevine MLO cistron candidates involved in susceptibility to powdery mildew. Funct Plant Biol. 2008;35(12):1255–66.
-
Liu Q, Zhu H. Molecular development of the MLO gene family in Oryza sativa and their functional divergence. Gene. 2008;409(1–2):1–ten.
-
Pessina Southward, Pavan South, Catalano D, Gallotta A, Visser R, Bai Y, et al. Characterization of the MLO cistron family in Rosaceae and factor expression assay in Malus domestica. BMC Genomics. 2014;fifteen(1):618.
-
Zhou SJ, Jing Z, Shi JL. Genome-wide identification, characterization, and expression analysis of the MLO cistron family in Cucumis sativus. Genet Mol Res. 2013;12(four):6565–78.
-
Singh VK, Singh AK, Chand R, Singh BD. Genome broad analysis of disease resistance mlo cistron family in sorghum [Sorghum bicolor (l.) Moench]. J Plant Genom. 2012;2(1):eighteen–27.
-
Pavan S, Jacobsen E, Visser RGF, Bai Y. Loss of susceptibility as a novel convenance strategy for durable and wide-spectrum resistance. Mol Breed. 2009;25(i):1–12.
-
Büschges R, Hollricher Chiliad, Panstruga R, Simons M, Wolter Grand, Frijters A, et al. The barley Mlo cistron: A novel control chemical element of plant pathogen resistance. Cell. 1997;88(v):695–705.
-
Panstruga R. Discovery of novel conserved peptide domains past ortholog comparison within plant multi-protein families. Plant Mol Biol. 2005;59(three):485–500.
-
Bai Y, Pavan Due south, Zheng Z, Zappel NF, Reinstädler A, Lotti C, et al. Naturally occurring broad-spectrum powdery mildew resistance in a Central American lycopersicon esculentum accession is caused by loss of Mlo part. Mol Establish-Microbe Collaborate. 2008;21(1):xxx–9.
-
Consonni C, Humphry ME, Hartmann HA, Livaja Yard, Durner J, Westphal L, et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat Genet. 2006;38(6):716–twenty.
-
Elliott C, Müller J, Miklis M, Bhat RA, Schulze-Lefert P, Panstruga R. Conserved extracellular cysteine residues and cytoplasmic loop-loop interplay are required for functionality of the heptahelical MLO protein. Biochem J. 2005;385(1):243–54.
-
Humphry K, Reinstädler A, Ivanov S, Bisseling T, Panstruga R. Durable broad-spectrum powdery mildew resistance in pea er1 plants is conferred by natural loss-of-function mutations in PsMLO1. Mol Establish Pathol. 2011;12(9):866–78.
-
Pavan Southward, Schiavulli A, Appiano Thousand, Marcotrigiano AR, Cillo F, Visser RGF, et al. Pea powdery mildew er1 resistance is associated to loss-of-function mutations at a MLO homologous locus. Theor Appl Genet. 2011;123(8):1425–31.
-
Várallyay É, Giczey Thousand, Burgyán J. Virus-induced cistron silencing of Mlo genes induces powdery mildew resistance in Triticum aestivum. Arch Virol. 2012;157(7):1345–50.
-
Zheng Z, Nonomura T, Appiano M, Pavan Due south, Matsuda Y, Toyoda H, Wolters AMA, Visser RGF, Bai Y: Loss of Office in Mlo Orthologs Reduces Susceptibility of Pepper and Lycopersicon esculentum to Powdery Mildew Affliction Caused by Leveillula taurica. PLoS ONE 2013, 8(7). ten.1371/periodical.pone.0070723
-
Appiano Thou, Pavan Southward, Catalano D, Zheng Z, Bracuto V, Lotti C, et al. Identification of candidate MLO powdery mildew susceptibility genes in cultivated Solanaceae and functional characterization of tobacco NtMLO1. Transgenic Res. 2015;24:1–12.
-
Peterhansel C, Freialdenhoven A, Kurth J, Kolsch R, Schulze-Lefert P. Interaction Analyses of Genes Required for Resistance Responses to Powdery Mildew in Barley Reveal Distinct Pathways Leading to Leaf Prison cell Decease. Establish Prison cell. 1997;9(8):1397–409.
-
Lipka Five, Dittgen J, Bednarek P, Bhat R, Wiermer 1000, Stein M, et al. Pre- and Postinvasion Defenses Both Contribute to Nonhost Resistance in Arabidopsis. Science. 2005;310(5751):1180–3.
-
Devoto A, Piffanelli P, Nilsson I, Wallin E, Panstruga R, Von Heijne G, et al. Topology, subcellular localization, and sequence diversity of the Mlo family in plants. J Biol Chem. 1999;274(49):34993–5004.
-
Müller J, Piffanelli P, Devoto A, Miklis Chiliad, Elliott C, Ortmann B, et al. Conserved ERAD-like quality control of a found polytopic membrane poly peptide. Plant Jail cell. 2005;17(one):149–63.
-
Panstruga R, Molina-Cano JL, Reinstädler A, Müller J. Molecular label of mlo mutants in N American two- and six-rowed malting barley cultivars. Mol Plant Pathol. 2005;6(3):315–20.
-
Piffanelli P, Zhou F, Casais C, Orme J, Jarosch B, Schaffrath U, et al. The barley MLO modulator of defense and cell decease is responsive to biotic and abiotic stress stimuli. Plant Physiol. 2002;129(three):1076–85.
-
Reinstädler A, Müller J, Czembor JH, Piffanelli P, Panstruga R. Novel induced mlo mutant alleles in combination with site-directed mutagenesis reveal functionally of import domains in the heptahelical barley Mlo protein. BMC Plant Biol. 2010;ten:31.
-
Wiberg A. Sources of resistance to powdery mildew in barley. Hereditas. 1974;78(1):1–40.
-
McCallum CM, Comai L, Greene EA, Henikoff S. Targeting Induced Local Lesions IN Genomes (TILLING) for Plant Functional Genomics. Plant Physiol. 2000;123(2):439–42.
-
Gaj T, Gersbach CA, Barbas Iii CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405. vii.
-
Terns RM, Terns MP. CRISPR-based technologies: prokaryotic defence weapons repurposed. Trends Genet. 2014;30(3):111–viii.
-
Kearse K, Moir R, Wilson A, Stones-Havas Southward, Cheung M, Sturrock S, et al. Geneious Bones: An integrated and extendable desktop software platform for the organization and analysis of sequence information. Bioinformatics. 2012;28(12):1647–9.
-
Pavan S, Zheng Z, Borisova Chiliad, Van Den Berg P, Lotti C, De Giovanni C, et al. Map- vs. homology-based cloning for the recessive gene ol-ii conferring resistance to tomato powdery mildew. Euphytica. 2008;162(1):91–8.
-
Anker C, Niks R. Prehaustorial resistance to the wheat foliage rust fungus, Puccinia triticina, in Triticum monococcum (s.south.). Euphytica. 2001;117(3):209–15.
Acknowledgements
We acknowledge Dr. Henk Schouten, Dr. Anne-Marie Wolters and Dr. Rients Niks for critical reading and valuable suggestions during the grooming of the manuscript. Nosotros also acknowledge Romero Cynara for her assist for inoculation with Blumeria graminis f.sp. hordei. The work of MA, CL, LR and SP were supported by the Italian Ministry building of Academy and Research through the GenHort PON R&C project.
Author information
Affiliations
Respective authors
Boosted data
Competing interest
The authors declare no competing interest.
Authors' contributions
MA developed transgenic plants, performed disease tests and was involved in experimental design, interpretation of results and manuscript drafting. MISM performed histological analyses. ZZ prepared overexpression constructs for transgenic complementation assays. DC, CL, RGFV, LR and YB were involved in experimental design and disquisitional revision of the manuscript. SP performed evolutionary analyses and was involved in experimental blueprint, interpretation of results and manuscript drafting. All authors read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
Codons under significant negative selection in PM susceptibility genes. Codon numbers refer to positions in the alignment of nine dicot MLO genes (AtMLO2, AtMLO6, AtMLO12, PsMLO1, MtMLO1, LjMLO1, CaMLO2, SlMLO1, NtMLO1) experimentally shown to human activity as powdery mildew susceptibility genes. Amino acid residues respective to each codon in barley HvMLO and pea PsMLO1 are indicated. For each residue, localization in any of the MLO protein domains, including seven transmembrane (TM) regions, three extracellular loops (East), three intracellular (I) loops, the N-terminus and the C-terminus, is indicated. Codons marked in bold are translated into grade-specific residues. The threshold p-value was 0.1, representing the default value for Single-likelihood Ancestor Counting (SLAC) analysis implemented by the Datamonkey web server. (DOCX 30 kb)
Additional file two: Figure S1.
Expression levels of PsMLO1 and HvMLO after transformation. Panel A) and console B) show the expression of PsMLO1 and HvMLO in nineteen and 20 T1 individuals, respectively, which were obtained by the transformation of the tomato plant mutant line Slmlo1, harboring a loss-of-function mutation of the endogenous SlMLO1 gene. Asterisks indicate T1 individuals selected for self-pollination and the evolution of Tii families. (PDF 173 kb)
Additional file 3: Figure S2.
Furnishings of transgenic overexpression of pea PsMLO1 and barley HvMLO in the tomato mutant line Slmlo1. Boilerplate disease index (DI) values and phenotypes are referred to transgenic plants of two additional Ttwo families segregating for PsMLO1 [35S::PsMLO1-4 and 35S::PsMLO1-6, panel a) and b)] and two additional Tii families segregating for HvMLO [35S::HvMLO-10 and 35S::HvMLO-xv), console c) and d)]. Data relative to the Slmlo1 mutant line, used equally genetic background for transformation, and not-transgenic plants of three Ttwo families for each overexpression construct (35S::PsMLO1_(−) and 35S::HvMLO_(−)) are also shown. (PDF 304 kb)
Additional file 4: Table S2.
Primer pairs used in this study. (DOCX 14 kb)
Rights and permissions
Open up Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted utilise, distribution, and reproduction in whatever medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and point if changes were made. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/ane.0/) applies to the information made bachelor in this article, unless otherwise stated.
Reprints and Permissions
Near this article
Cite this article
Appiano, Chiliad., Catalano, D., Santillán Martínez, M. et al. Monocot and dicot MLO powdery mildew susceptibility factors are functionally conserved in spite of the evolution of course-specific molecular features. BMC Plant Biol 15, 257 (2015). https://doi.org/x.1186/s12870-015-0639-6
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12870-015-0639-half-dozen
Keywords
- MLO
- Powdery mildew
- Angiosperms
- Evolution
- Plant breeding
Source: https://bmcplantbiol.biomedcentral.com/articles/10.1186/s12870-015-0639-6
0 Response to "How Do You Know Monocot and Dicot Are Basic or Acidic? After Pcr"
Postar um comentário